I recently published the first video in an animated series I'm creating about human biology. It's targeted at as broad of an audience as I could make it, and doesn't make too many assumptions about prior knowledge. If you haven't already watched it, I highly recommend giving it a look.
The purpose of this blog post is to give a little peak behind the curtains to see some "making of" material, and to house the references I used to support the information in the video.
For the animation, I used Houdini almost exclusively, rendered with Redshift, comped in Blackmagic Design Fusion, and edited and mixed in DaVinci Resolve.
Houdini is a very interesting and unique 3D application, in that almost everything is created in a procedural nature, which means that you set up "rules" for how things are created, instead of creating each thing individually. This was very helpful for creating the Periodic Table which featured in the video.
I started by laying out a grid of points that would determine where the elements would end up.
Computers start counting from zero. Adjust accordingly.
Then I imported a spreadsheet of data about the elements, including symbol, name, element number, and atomic radii. Then I mapped that data to the grid of points.
If there are any typos, it’s wikipedia’s fault.
Then, when I had to create animation, for example when pulling out a highlighted element, I was able to set up a "selector" which allowed similar animations to be repeated quite simply.
Oxygen comin’ atcha!
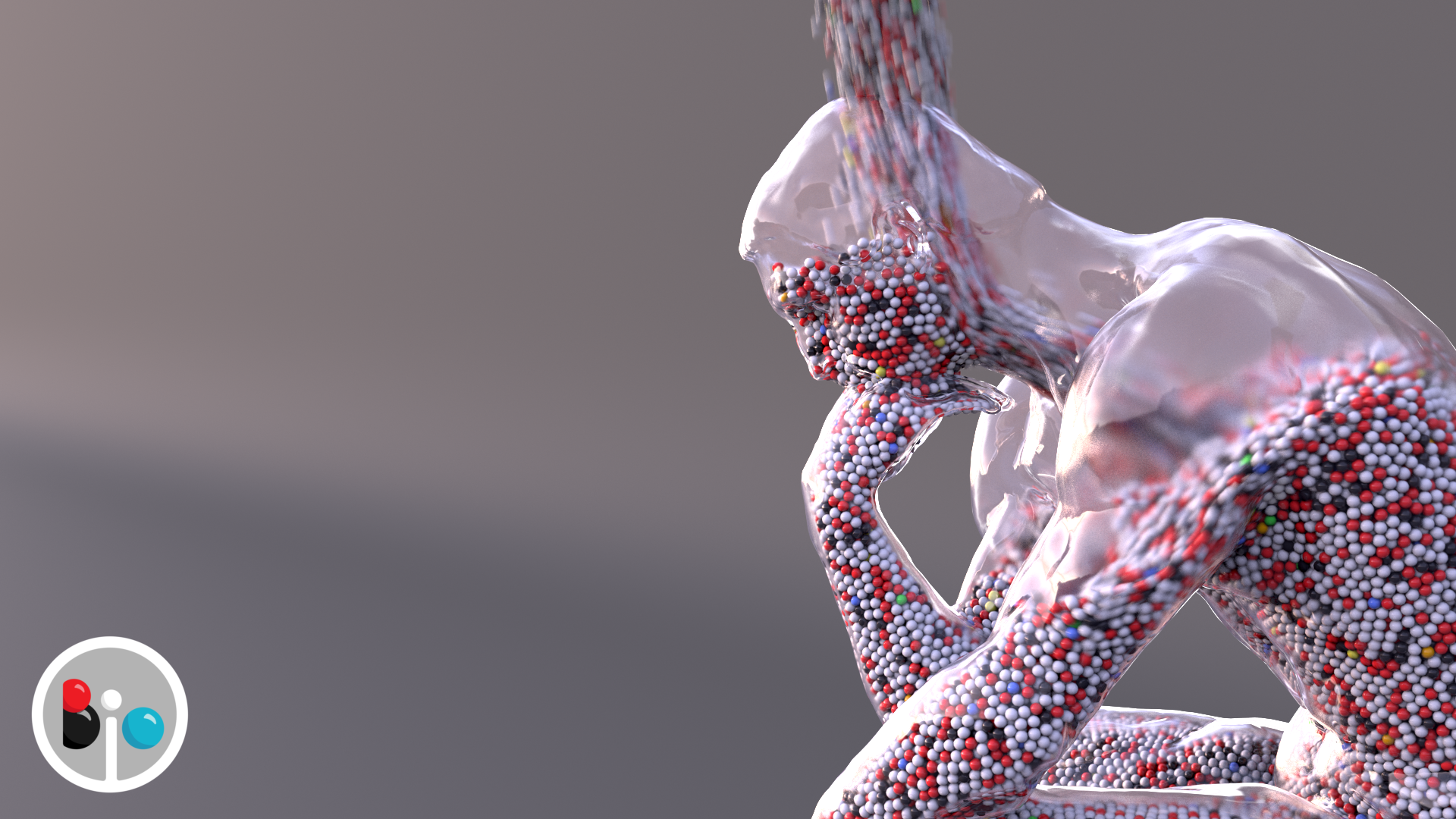
Probably the most involved shot in the video is the "Thinker" being filled with colored atoms pouring in. I was able to find a 3D scan of the sculpture by Rodin, and after some cleanup and retopology, it was ready to fill. Of course things usually don't work out immediately.
I’ve certainly felt like this before.
But eventually I got the spheres filling the statue, albeit with some leakage.
The leaking was a lot worse in other iterations.
Ultimately I set up a rule that if a sphere leaked outside the statue, it was killed from the simulation, so it doesn't show up.
Not quite right.
And rendering is its own challenge. I started using a very basic type of sphere geometry, but it turns out it was designed for very small particles, and so I had to switch to a more complex kind of sphere (skimming over the details here) to avoid artifacts like this where the spheres and glass statue intersect.
I'm pretty pleased with how the final shower of atoms turned out (at 3:17 in the video). Of course I had to search for some "rainstick" sound effects to fit with the visuals.
And now for something completely different.
You may not know that I've spent quite some time in an academic setting considering how one might present the references that inform different aspects of an illustration, video or animation, from narration to objects, behaviours, and even colors. I contributed to a publication in Nature Methods on the topic: http://rdcu.be/doo5.
Needless to say, dumping references here without linking bidirectionally to specific points in the video is a failure in many respects, but it's better than nothing, and I plan to improve the way in which I present this kind of information as time progresses. Maybe I should re-read my article. Also, almost certainly this is an incomplete list. I’m pretty sure there were lots of wikipedia pages I used at various points and didn’t go through the trouble of tracking down primary references. Lots of room for improvement.
References:
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2002). Molecular Biology of the Cell (4th ed.). Garland Science.
Bentley, G., Dodson, E., Dodson, G., Hodgkin, D., & Mercola, D. (1976). Structure of insulin in 4-zinc insulin. Nature, 261, 166–168. https://doi.org/10.2210/pdb1zni/pdb
Biomolecule | biology. (n.d.). Retrieved May 8, 2019, from Encyclopedia Britannica website: https://www.britannica.com/science/biomolecule
Bruce, A., Andersson, M., Arvidsson, B., & Isaksson, B. (1980). Body composition. Prediction of normal body potassium, body water and body fat in adults on the basis of body height, body weight and age. Scandinavian Journal of Clinical and Laboratory Investigation, 40(5), 461–473. https://doi.org/10.3109/00365518009101869
Campbell, N. A., & Reece, J. B. (2001). Biology, 6th Edition (6 edition). San Francisco: Benjamin Cummings.
Drew, H. R., Wing, R. M., Takano, T., Broka, C., Tanaka, S., Itakura, K., & Dickerson, R. E. (1981). Structure of a B-DNA dodecamer: Conformation and dynamics. Proc.Natl.Acad.Sci.USA, 78, 2179–2183. https://doi.org/10.2210/pdb1bna/pdb
Emsley. (1998). The Elements. Clarendon Press.
Fomon, S. J., & Nelson, S. E. (2002). BODY COMPOSITION OF THE MALE AND FEMALE REFERENCE INFANTS. Annual Review of Nutrition, 22(1), 1–17. https://doi.org/10.1146/annurev.nutr.22.111401.145049
Forbes, R. M., Cooper, A. R., & Mitchell, H. H. (n.d.). OF THE ADULT HUMAN BODY AS BY CHEMICAL ANALYSIS. 9.
Freitas, R. A. (1998). 3.1 Human Body Chemical Composition. Retrieved May 7, 2019, from Nanomedicine website: https://foresight.org/Nanomedicine/Ch03_1.php
Goodsell, D. S. (2005). Visual Methods from Atoms to Cells. Structure, 13(3), 347–354. https://doi.org/10.1016/j.str.2005.01.012
Harris, L. J., Larson, S. B., Hasel, K. W., & McPherson, A. (1997). Refined structure of an intact IgG2a monoclonal antibody. Biochemistry, 36, 1581–1597. https://doi.org/10.2210/pdb1igt/pdb
International Year Periodic Table 2019 | IYPT 2019. (n.d.). Retrieved May 8, 2019, from The International Year of the Periodic Table website: https://www.iypt2019.org/
JMOL Color Table. (n.d.). Retrieved May 8, 2019, from Jmol website: http://jmol.sourceforge.net/jscolors/
Koltun, W. L. (1965). Precision space-filling atomic models. Biopolymers, 3(6), 665–679. https://doi.org/10.1002/bip.360030606
L, N. D., Lehninger, A. L., Nelson, D. L., Cox, M. M., Cox, U. M. M., & Cox, M. M. (2005). Lehninger Principles of Biochemistry. W. H. Freeman.
Natchiar, S. K., Myasnikov, A. G., Kratzat, H., Hazemann, I., & Klaholz, B. P. (2017). Visualization of chemical modifications in the human 80S ribosome structure. Nature, 551, 472–477. https://doi.org/10.2210/pdb6qzp/pdb
Otterbein, L. R., Graceffa, P., & Dominguez, R. (2001). The crystal structure of uncomplexed actin in the ADP state. Science, 293, 708–711. https://doi.org/10.2210/pdb1j6z/pdb
Pullman, B. (2001). The Atom in the History of Human Thought. Oxford University Press.
RCSB Protein Data Bank. (n.d.-a). RCSB PDB - ale Ligand Summary Page L-EPINEPHRINE. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/ale
RCSB Protein Data Bank. (n.d.-b). RCSB PDB - asc Ligand Summary Page ASCORBIC ACID. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/asc
RCSB Protein Data Bank. (n.d.-c). RCSB PDB - atp Ligand Summary Page ADENOSINE-5’-TRIPHOSPHATE. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/atp
RCSB Protein Data Bank. (n.d.-d). RCSB PDB - cys Ligand Summary Page CYSTEINE. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/cys
RCSB Protein Data Bank. (n.d.-e). RCSB PDB - glc Ligand Summary Page ALPHA-D-GLUCOSE. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/glc
RCSB Protein Data Bank. (n.d.-f). RCSB PDB - gly Ligand Summary Page GLYCINE. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/gly
RCSB Protein Data Bank. (n.d.-g). RCSB PDB - ldp Ligand Summary Page L-DOPAMINE. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/ldp
RCSB Protein Data Bank. (n.d.-h). RCSB PDB - nad Ligand Summary Page NICOTINAMIDE-ADENINE-DINUCLEOTIDE. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/nad
RCSB Protein Data Bank. (n.d.-i). RCSB PDB - oli Ligand Summary Page. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/oli
RCSB Protein Data Bank. (n.d.-j). RCSB PDB - pyr Ligand Summary Page PYRUVIC ACID. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/pyr
RCSB Protein Data Bank. (n.d.-k). RCSB PDB - ser Ligand Summary Page SERINE. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/ser
RCSB Protein Data Bank. (n.d.-l). RCSB PDB - tes Ligand Summary Page TESTOSTERONE. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/tes
RCSB Protein Data Bank. (n.d.-m). RCSB PDB - trp Ligand Summary Page TRYPTOPHAN. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/trp
RCSB Protein Data Bank. (n.d.-n). RCSB PDB - viv Ligand Summary Page (2R)-2,5,7,8-TETRAMETHYL-2-[(4R,8R)-4,8,12-TRIMETHYLTRIDECYL]CHROMAN-6-OL. Retrieved June 25, 2019, from RCSB PDB website: https://www.rcsb.org/ligand/viv
Rutherford, P. E. F. R. S. (1911). LXXIX. The scattering of α and β particles by matter and the structure of the atom. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 21(125), 669–688. https://doi.org/10.1080/14786440508637080
Slater, J. C. (1964). Atomic Radii in Crystals. The Journal of Chemical Physics, 41(10), 3199–3204. https://doi.org/10.1063/1.1725697
Tame, J. R., & Vallone, B. (2000). The structures of deoxy human haemoglobin and the mutant Hb Tyralpha42His at 120 K. Acta Crystallogr.,Sect.D, 56, 805–811. https://doi.org/10.2210/pdb1a3n/pdb
Thomson M. A., J. J. (1897). XL. Cathode Rays. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 44(269), 293–316. https://doi.org/10.1080/14786449708621070
Wieser, M. E., Holden, N., Coplen, T. B., Böhlke, J. K., Berglund, M., Brand, W. A., … Zhu, X.-K. (2013). Atomic weights of the elements 2011 (IUPAC Technical Report). Pure and Applied Chemistry, 85(5), 1047–1078. https://doi.org/10.1351/PAC-REP-13-03-02